Mining Critical Materials from Seawater and Brine
Technology Roadmap Sections and Deliverables
This is a technology roadmap for:
- 4AIE - Adsorption and Ion Exchange
Roadmap Overview
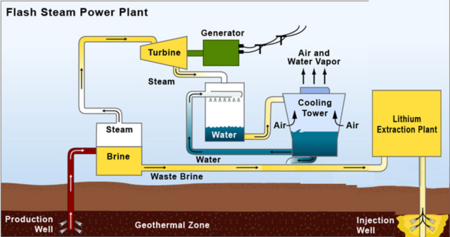
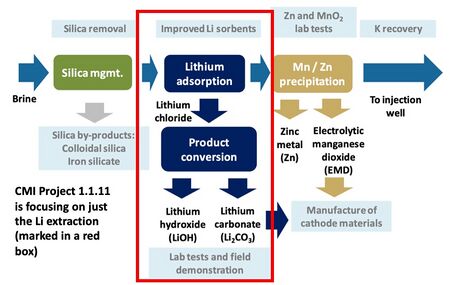
There are a variety of potential sources of lithium globally including minerals (e.g. clay, seawater, etc.); lithium-ion battery recycling; and saltwater brines (e.g. geothermal, continental, salt lakes, oil fields, etc.). We are focused on exploring lithium extraction from geothermal brines for a variety of reasons. One is that the concentration of lithium is higher in geothermal brines (approximately 300 ppm) than in other potential sources of lithium such as saltwater. Further, the Salton Sea in Southern California hosts a high concentration of lithium and is an attractive natural resource for the United States to take advantage of. Roughly 13 geothermal plants operate in this region with a combined electric generating capacity of 375 megawatts (MW), which together generate enough brine to recover a potential 200 metric tons of lithium per year - enough to supply the global demand for lithium. We are roadmapping a sorbent technology that extracts lithium from geothermal brines with a focus on how this technology can be applied to geothermal plants in Southern California. The two graphics below break out the schematic and R&D boundaries for the sorbent technology.
Concept of mineral extraction plant utilizing post-power production, pre-injection geothermal brine.<ref>Paranthaman, M. P., Li, L., Luo, J., Hoke, T., Ucar, H., Moyer, B. A., & Harrison, S. (2017). Recovery of lithium from geothermal brine with lithium–aluminum layered double hydroxide chloride sorbents. Environmental Science & Technology, 51(22), 13481–13486.</ref>
Schematic of R&D tasks.<ref>Paranthaman, M. P. “Lithium extraction from geothermal brine solution.” Webinar presented by the U.S. Department of Energy Oak Ridge National Laboratory.” 2018. Available at: https://iastate.app.box.com/v/cmi-webinar-november-2018</ref>
Design Structure Matrix (DSM) Allocation
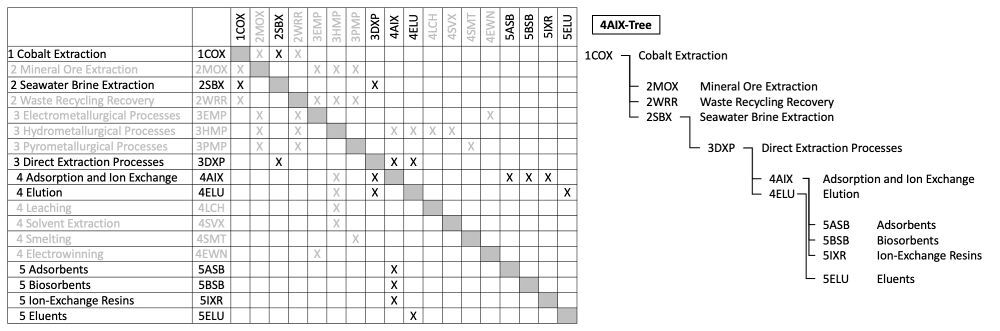
The 4AIX tree extracted from the DSM above shows that seawater brine extraction (2SBX) is a novel method of cobalt extraction (1COX), in comparison to the conventional mineral ore extraction (2MOX) and the emerging waste recycling recovery (2WRR) methods. It requires some enabling technologies in the direct extraction processes (3DXP). Innovation in adsorption and ion exchange (4AIX), as well as elution (4ELU), are some of these promising Level 4 technologies. Within the focus of 4AIX in this roadmap, there is a wide range of promising materials including adsorbents (5ASB), biosorbents (5BSB), and ion-exchange resins (5IXR).
Roadmap Model using OPM
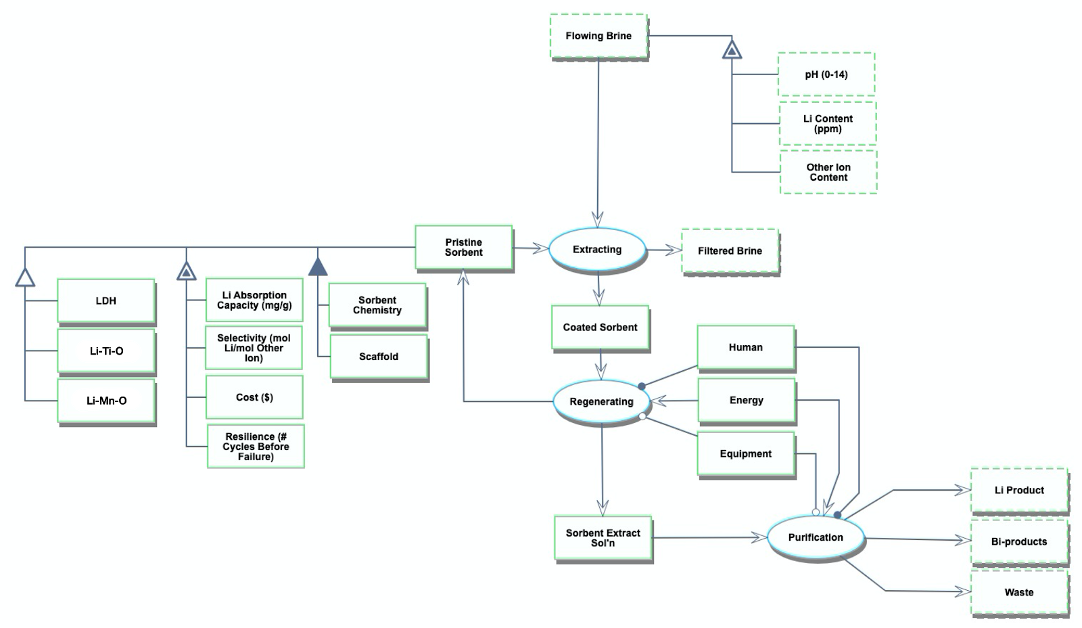
The Object-Process-Diagram (OPD) of the 4AIE roadmap is shown in the figure below. This diagram captures the main object of the roadmap (sorbent), its various instances including Lithium Manganese Oxides (Li-Mn-O), Lithium Titanium Oxides (Li-Ti-O), Lithium Aluminum Layered Double Hydroxide (LDH) Chloride; and its characterization by Figures of Merit (FOMs).
Figures of Merit
| FOM Name | Unit | Description |
|---|---|---|
| Adsorption capacity | [mg/g] |
|
| Selectivity | [dmnl] |
|
| Lithium Production Cost | [$/kg] |
|
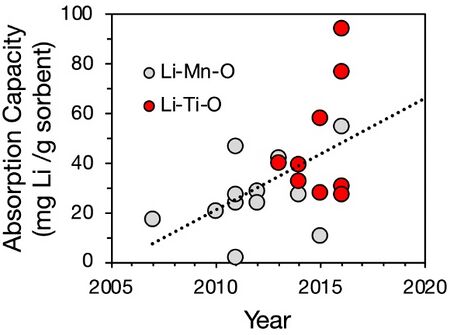
We gathered reported data from 21 peer-reviewed publications within the Li brine extraction literature to generate a plot of sorption capacity. Note that we provide data for only 2 of the 3 sorbents identified in our roadmap OPM (Li-Mn-O and Li-Ti-O), as there are few reports of sorbent capacity for lithium aluminum layered double hydroxide chloride (LDH) sorbent.
Adsorption capacities of major sorbents.<ref>Li, L., Deshmane, V. G., Paranthaman, M. P., Bhave, R., Moyer, B. A., & Harrison, S. (2018). Lithium recovery from aqueous resources and batteries: A brief review. Johnson Matthey Technology Review, 62(2), 161–176.</ref>
Alignment with Company Strategic Drivers
Our company provides leading-edge adsorbates within ion-sorption systems submerged in seawater. Currently, we identify the most important FOMs driving strategy as capacity (mg adsorbate/g sorbent) and degradation rate (% reduction in capacity per load/unload cycle). This can be combined into an overall FOM of mg adsorbate/g sorbent over the lifetime of the adsorbate. This is calculated as:
<math display="block">\begin{align}
\text{lifetime capacity}=C_T=\sum_{n=1}^NC{}_i(1-{}r_r)^{n-1}
\end{align}</math>
where <math display="block">\begin{align}n\end{align}</math> is the cycle number, <math display="block">\begin{align}N\end{align}</math> is the total number of cycles, and <math display="block">\begin{align}r_r\end{align}</math> is the degradation rate per cycle, and <math display="block">\begin{align}C_i\end{align}</math> is the initial sorbent capacity.
Below, we provide a set of targets based on a strategic driver involving lifetime capacity (<math display="block">\begin{align}C_T\end{align}</math>).
| Strategic Driver | Alignment and Target |
|---|---|
| To achieve competitive economics with other forms of Co extraction, <math display="block">\begin{align}C_T\end{align}</math> for our technology needs to be 680 mg/g (4x single-cycle nZVI capacity) | Produce a field-deployable adsorbent that can harvest Co for 4 cycles with 0 degradation, or 6 cycles with a constant degradation rate less than that permitted by lifetime capacity equation (7.5%) |
| Infrastructure of Co-harvesting system cannot interfere with sorbent capacity over the course of sorbent lifetime. |
Positioning of Company vs. Competition
See below for a chart outlining the current state of play for several key figures of merit (FOM) for sorbents designed to extract cobalt from seawater. Please note that for some figures of merit where data was either limited or difficult to compile, figures for seawater extraction of uranium were used instead because of their analogous project scopes and figures of merit.
| FOM | Units | Level Achieved | Target |
|---|---|---|---|
| Adsorption Capacity | mg Co/g sorbent | 166 (SiO2-Gly)
172 (nZVI) 196 (Mesosponge) 262 (Hydrogel) |
As high as possible |
| Selectivity | Unitless | High (nZVI) | High |
| Unit Production Cost | $/kg Co | N/A | <USD36.06
(current spot price of cobalt) |
| Reusability | Number of Reuse Cycles | 20 (Uranium) | As many as possible |
| Durability | % retained per re-use | 5% (Uranium) | As high as possible |
Technical Model
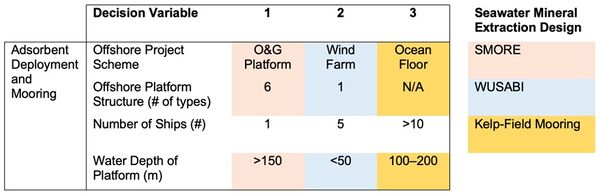
Below is an initial morphological matrix for different seawater mineral extraction designs. We focused this initial morphological matrix on the adsorbent deployment and mooring setup because the technology for our roadmap is very component level, so there aren’t many decision variables aside from the choice of adsorbent. Accordingly, we have gone a level up for our morphological matrix where other key decision variables impact cobalt extraction schematics. Below is a matrix with an initial set of key design variables impacting the efficiency and production cost of cobalt extraction from seawater. Because there is such little data available about seawater extraction of cobalt, much of this data is based on analogous offshore designs for harvesting uranium from seawater. Please note that cells that are unshaded are applicable for all 3 seawater mineral extraction designs.
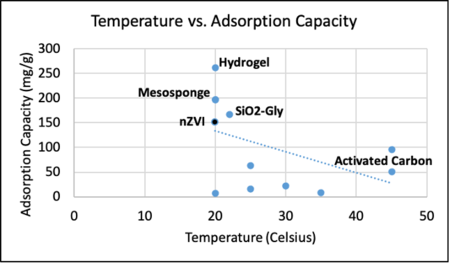
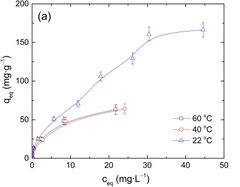
We also developed an initial snapshot of a key element in the tradespace for various sorbents that can extract cobalt from seawater. One parameter that impacts a sorbent’s adsorption capacity is temperature. This is a parameter impactful in the tradespace because seawater temperature varies by location and may impact the first decision variables listed in our morphological matrix - offshore platform scheme. In the United States, much of the offshore oil and gas infrastructure is located in the Gulf of Mexico. By contrast, the only wind farm operating in the United States is off the coast of Rhode Island. The seawater temperature in each of these locations varies drastically and, as such, the tradeoff between adsorption capacity and seawater temperature is important to keep in mind in selecting a cobalt adsorbent. Project developers should select a sorbent towards the upper right corner of the graph below. We will explore further aspects of the tradespace in subsequent iterations of our technology roadmap.
Comparison of adsorption capacity for sorbents at different temperatures.<ref>Piątek, J., Bruin-Dickason, C. N., Jaworski, A., Chen, J., Budnyak, T., & Slabon, A. (2020). Glycine-functionalized silica as sorbent for cobalt(II) and nickel(II) recovery. Applied Surface Science, 530, 147299.</ref>
Financial Model
We create a financial model for a company moving into the Co seawater mining business. This is a public-private partnership in which the company agrees to reach at least 35% of U.S. Co consumption per year (~3000 tonnes/y) by year 5 of project launch. In return, the government will pay all capital expenses related to retrofitting 40 decommissioned oil rigs ($40M over 5 years) and the company can employ depreciation tax benefits on these capital expenditures (lifetime = 20 years).
Key assumptions for this model are in the below table:
| Assumption | Value |
|---|---|
| Operating Margin | 10% (initial)
25% (final) |
| Shells/rig | 11.2k |
| Mass sorbent/shell | 5 kg |
| Discount rate | 15% |
| Cobalt price | $30/kg (initial)
$50/kg (final) |
| Sorbent capacity | 172 g Co/kg sorbent |
| Tax Rate | 21% |
| CapEx of retrofit | $1M/rig |
| Sorption cycle time | 1 month |
| Uptime | 100% |
| Desorption Efficiency | 70% (initial)
90% (final) |
| Fixed Costs (manufacturing, salaries, benefits) | $5M/y |
| Payor for CapEx | Government |
| Tax benefits of depreciation | Provided to sorbent company at linear depreciation |
| Lifetime of rig | 20 years |
| Growth rate for US Co consumption | 2.5%/year |
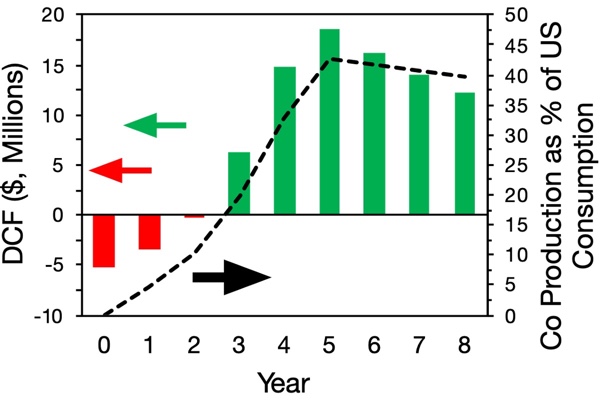
With these assumptions, we prepared the following discounted cash flow analysis for this project (see below). At a discount rate of 15%, the NPV for this project is $155M, implying that this project will produce value over its lifetime.
Key Publications, Presentations and Patents
Some of the key publications related to cobalt extraction from aqueous solutions:
While the Cooperative Patent Classification (CPC) has a subclass C22B, group 23/00 that covers the production of cobalt by a metallurgical process, the patents for the novel extraction of cobalt from seawater are scarce. Therefore, we searched for and found the following related patents of other metals, although most of them have already expired.
| Year | Patent No | Title | Claims Summary | Assignee | Status |
|---|---|---|---|---|---|
| 1986 | US4585627A | Process for the concentration of uranium from sea water | A process for the selective concentration of uranium from sea water through chemical accumulation on a solid adsorption medium, and subsequent elution with carbonate-containing sea water. | Kernforschungsanlage Julich GmbH | Expired |
| 1987 | SU1553538A1 | Cross-linked copolymer of tetravinyl ether of pentaerythritol and maleic anhydride as a sorbent of cobalt from acidic media | An invention that improves the adsorption capacity of cobalt up to 125 mg/g at pH 1–3. | - | Expired |
| 2019 | US10439200B2 | Ion exchange system for lithium extraction | A process for the extraction of lithium ions from a liquid source, by using porous beads coated with ion exchange particles. | Lilac Solutions Inc | Active |
List of R&T Projects and Prototypes
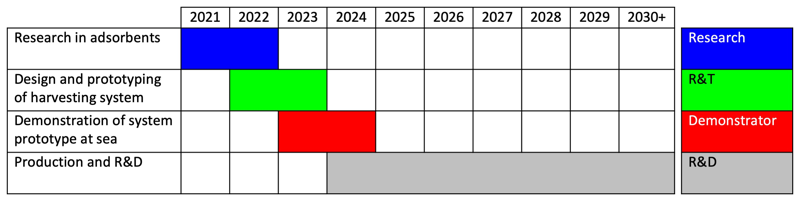
Given that seawater mining is only at the conceptual stage at present, the list of projects planned will be organized along the Technology Readiness Level (TRL) scale. The strategy is to advance from proof-of-concept from the laboratory to a demonstrator project within 5 years.
- To research adsorbents and related technologies: Further analytical and laboratory studies on selected candidates of adsorbent material, especially to investigate the primary FOMs of adsorption capacity and selectivity in seawater and other conditions (e.g. pH, temperature, contact time).
- To design and prototype the harvesting system: Embodiment of the adsorption and elution processes, designed in accordance with the characteristics of the adsorbent and resulting system requirements.
- To demonstrate the system prototype: Retrofit of an existing offshore structure<ref>M.N., Haji, and A.H., Slocum, “An offshore solution to cobalt shortages via adsorption-based harvesting from seawater”, Renewable and Sustainable Energy Reviews, vol. 105, pp. 301–309, 2019.</ref>, e.g. a decommissioned oil platform, with the demonstrator system as a testbed for pre-production.
Following production in 2025, R&D will focus on improving the operations as modeled in the NPV analysis, with the following projects:
- Increasing desorption efficiency from 70% to 90% in 4–5 years.
- Improving cost and increasing operating margin from 10% to 25% in 4–5 years, by enabling technologies e.g. faster elution process.
- Enhancing the safety and sustainability in handling cobalt due to its toxicity.
The following Gantt chart illustrates the key milestones:
Technology Strategy Statement
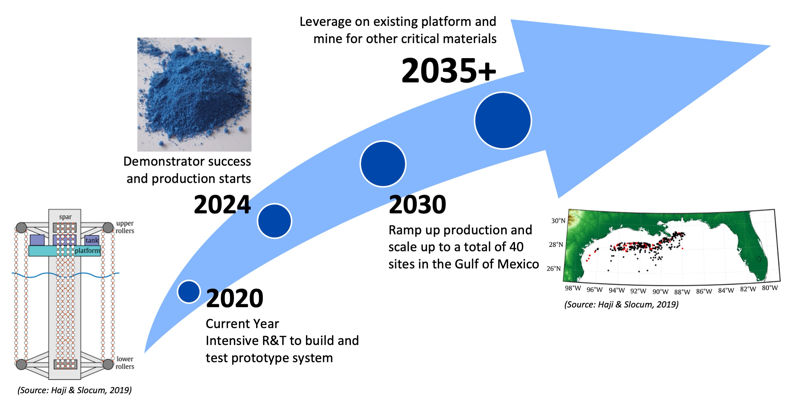
Our target is to develop a passive extraction system of cobalt from seawater with production planned to start in 2024. To achieve the target of meeting over 40% of US cobalt consumption in the next ten years, we will invest in a prototype development project of a harvesting system using nZVI as the adsorbent, which will lead to a demonstrator project and production start within five years, and a scaled-up operation by 2030.
References
<references />