Plasma-based Oxygen Generation in Space
Roadmap Creators: | Lanie McKinney | Roy Weinstock
Technology Roadmap Sections and Deliverables
This technology roadmap has the unique identifier:
2PBOGS - Plasma-based Oxygen Generation in Space
The number 2 denotes that this is a Level 2 technology roadmap at the product level. In reference to our technology, Level 1 encompasses all oxygen generation technologies that can be used in space. Level 3, the system level, could reference the plasma reactor configuration for conversion and Level 4, the subsystem level could represent the the kind of plasma source utilized.
Roadmap Overview
Oxygen generation in space is a critical enabling technology to support the development of human exploration of the Moon and Mars. NASA's Artemis program plans to head back to the Moon this decade in a phased approach to build up lunar activity, including a commercial and International presence, to serve as a proving ground for human missions to Mars. Increasingly longer stays off-world at destinations of increasing distance from Earth require technologies that recycle and utilize available resources, called in-situ resource utilization (ISRU), to make large mission architectures feasible and enable dramatic cost-savings. Notably, the most near-term large-scale oxygen demand will be driven by lunar lander propellant needs, like Starship and Blue Moon (both contracted by NASA as part of the Human Landing System). One projection estimates that the market valuation of the ISRU sector will reach 63$ billion by 2040, where 99% of the demand is driven by propellant [1]. There are three primary resources available in space to extract oxygen: H2O, CO2, and Regolith. Water and oxygen found in regolith are both available on the Moon and Mars, whereas CO2 is only found in the Mars environment.
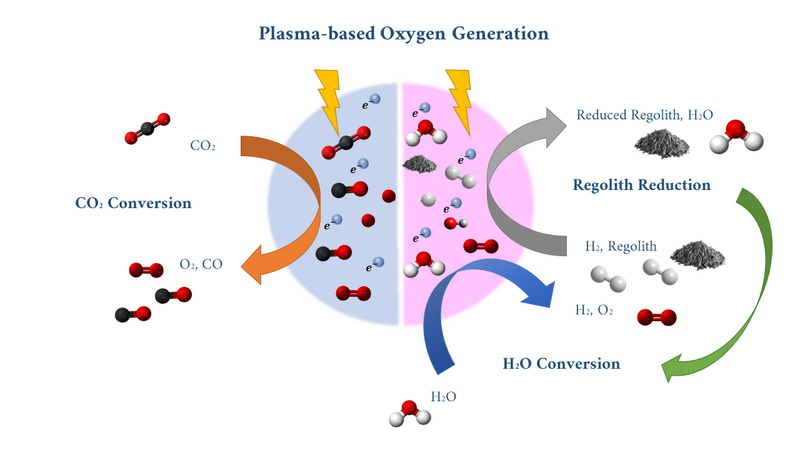
Non-thermal plasma reactors are emerging power-to-gas technologies with the potential to facilitate various chemical synthesis processes. In plasma-based systems, electrical power is used to ionize a feedstock gas, creating a highly reactive environment that leverages electron excitation chemistry to break stable molecular bonds and produce value-added products. Plasma reactors operate at non-equilibrium conditions, allowing for lower-temperature operation and instantaneous start-up, making them adaptable to intermittent power availability. Unlike other technologies, plasma reactors can process any feedstock, and have electrical settings and environmental conditions which can be tuned to optimize or favor particular products. Plasma reactors can reduce regolith and produce water, as well as convert water and CO2 into oxygen. This means that plasma-based conversion technologies are extensible to both near and far-term missions, and can be used where any of these resources are found on the Moon and Mars.
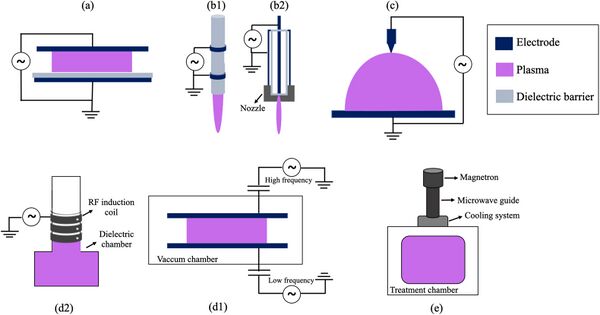
In the diagram above, plasma-based technologies potential for performing multiple chemical conversion processes relevant to available resources to produce oxygen are shown. In the diagram below, a few of the common plasma discharge reactor configurations are shown, including (a) dielectric barrier discharges, (b1) plasma jet, (c) Corona discharges, (d1) Radiofrequency Inductively coupled plasma, and (d2) AC-driven Capacitively coupled plasmas, and (e) Microwave plasmas [2].
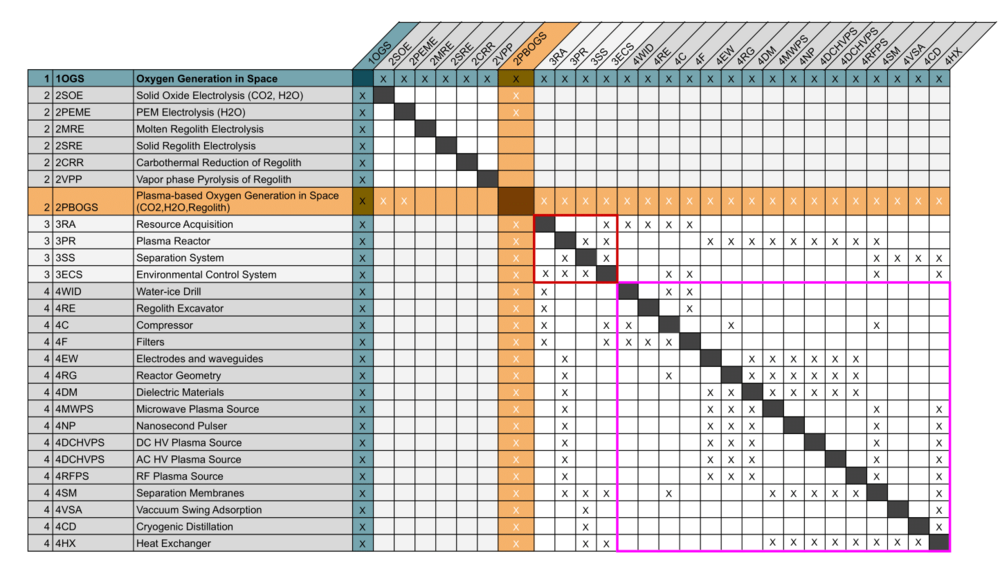
Design Structure Matrix (DSM) Allocation
The market level DSM for Oxygen Generation includes candidate technologies which can extract oxygen from a variety of resources available in space, namely CO2, water, and regolith, and shows that plasma-reactors can be combined with other conversion technologies for enhanced performance. At the system level, key enabling technologies are the resource acquisition system (3RA), the plasma reactor (3PR), separation system (3SS), and environmental control system (3ECS). At level 4, the enabling technologies include the acquisition systems needed to extract the different resources (4C, 4WID, 4RE), the systems to control the conditions of the input stream (4F, 4HX, 4C), 5 different kinds of plasma sources (3MWPS, 3NP, 3DCHVPS, 3ACHVPS, 3RFPS) which generate plasmas in different ways, 3 technologies related to reactor design (4RG, 4DM, 4EW), and 3 relevant kinds of separation technologies (3SM, 3VSA, 3CD). The DSM at this level clearly demonstrates the cluster of interdependencies around the selection of the the plasma source and elements of the overall reactor configuration (. Different combinations of level 4 elements, like the plasma source, reactor geometry, and separation subsystem may lead to novel performance.
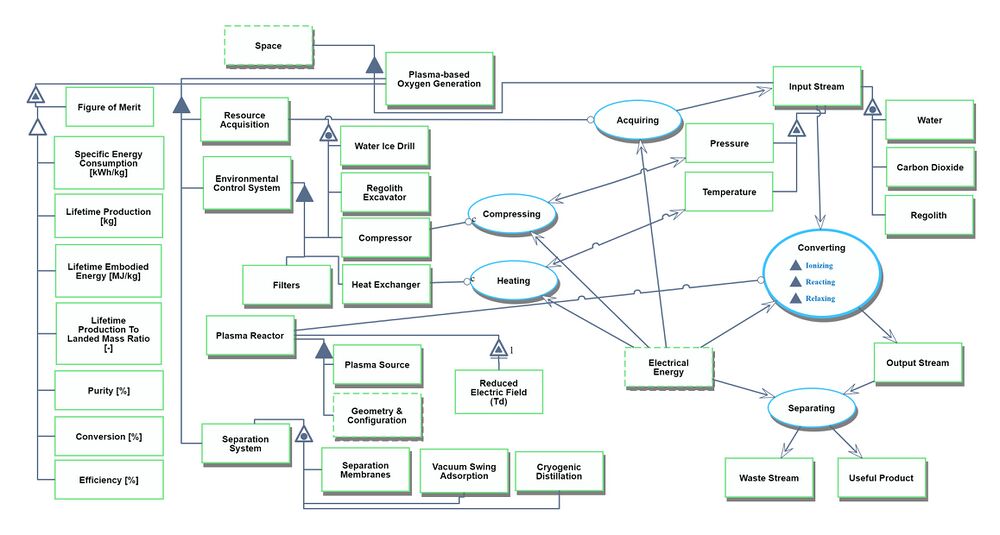
Roadmap Model using OPM
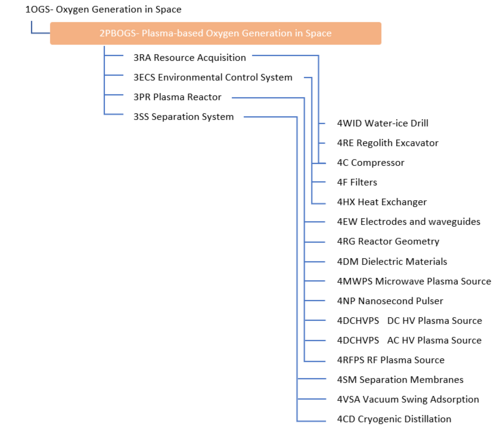
An Object-Process-Diagram (OPD) of the 2PBOGS roadmap is given in the figure below. This diagram captures the main product of the roadmap (level 2) and decomposes the product into four main systems (resource acquisition, environmental control, plasma reactor, and separation) corresponding to level 3 in the DSM and displays the figures of merit (FOMs).
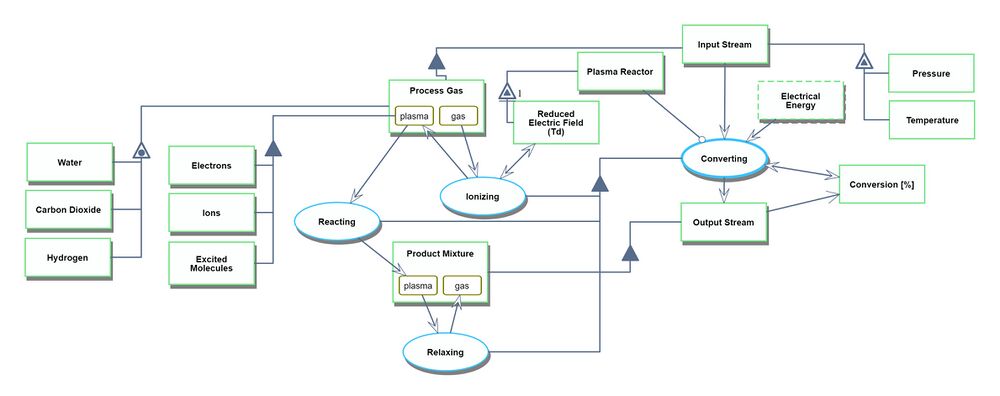
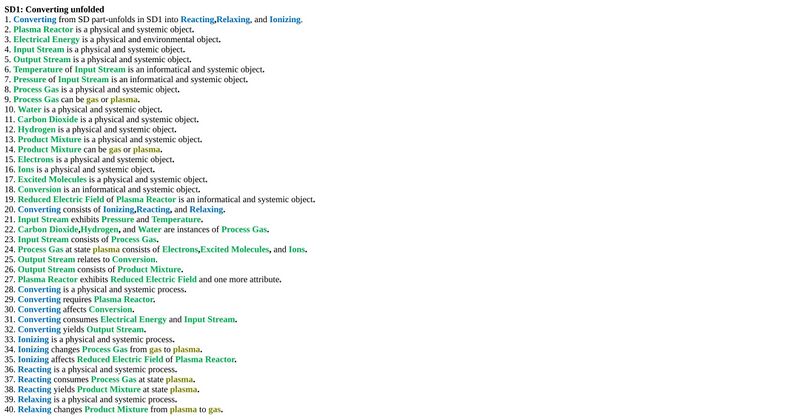
Unfolding the converting process at level SD1
An Object-Process-Language (OPL) description of the roadmap scope is auto-generated and given below. It reflects the same content as the previous figure, but in a formal natural language.
Figures of Merit
The table below show a list of FOMs that can be used to assess Space Resource Generation Technologies.
| Figure of Merit | Unit | Description |
|---|---|---|
| Lifetime Produced | kg | resource mass produced over the lifetime of the system (level 1) |
| Lifetime Embodied Energy | MJ/kg | the thermodynamic sum of past, present and future work required to create, operate, maintain and decommission a system (level 1) |
| Lifetime Produced vs Landed Mass Ratio | - | the ratio of total mass produced over the system lifetime compared to the landed mass required for it's lifetime (level 1) |
| Specific Energy Consumption | kWh/kg | total energy required to produce a kg of product (level 1) |
| Conversion | % | ratio of useful product generated to the total available input reactant (level 2) |
| Efficiency | % | ratio of theoretical energy required for chemical conversion to the total energy consumed by the process (level 2) |
| Purity | % | percentage of the target product in separated product stream (level 1) |
Lifetime Mass Produced
This metric captures the true benefit of a resource generation technology, the total mass a system can produce over it's lifetime.
Lifetime Embodied Energy (LEE)
This FOM is defined as “...the thermodynamic sum of past, present and future work required to create, operate, maintain and decommission a system, including appropriate shares of indirect contributions from upstream systems as well as from other systems in a system-of-systems [1].” This metric has been shown to reproduce results from Equivalent System Mass analyses, the industry standard for trading advanced life support systems, while decoupling from launch mass (which is becoming an outdated cost proxy). This FOM utilizes the total energy required across the whole architecture's lifetime as a proxy for cost, and normalizes it with the lifetime mass produced. This (cost/benefit) is an extremely relevant metric to the space industry for determining what the real cost of developing infrastructure and operating a space resource generation technology over long time periods to capture economies of scale and identify promising business use cases.
Lifetime Produced vs Landed Mass Ratio
It can be useful to contextualize lifetime production in terms of total system mass landed on the planetary surface, which can be expressed as a ratio of total product over landed mass. A recommended best practice is to compare this benefit/cost metric with the inverse of the physics-based metric LEE, as they capture aspects of the architecture in different ways. As the space industry evolves and launch costs become increasingly decoupled from total system cost, this kind of metric may become increasingly outdated.
Specific Energy Consumption
This FOM describes the energy required by the technology per kg of value-added output produced on the surface. It is an energy efficiency metric which can allow comparison between different technologies which may use different chemical pathways. With peak or maximum production rate, this FOM’s can be multiplied to imply a power requirement. Though similar in units to LEE, this metric describes the technology performance as opposed to the full architecture performance.
Purity
This FOM describes how pure the useful product stream is after separation of the output stream, which is an evaluation of conversion and separation performance. There are also different purity targets when oxygen is used either as propellant or life-support.
Conversion
This is a commonly reported metric for describing the % of an input stream converted into products.
Efficiency
Also known as first-law efficiency, this is a commonly reported metric for describing a conversion technology's comparison to the theoretical energy required to perform the targeted chemical transformation. It is a ratio of the theoretical energy consumption and the actual specific energy consumption of the technology and given in %.
Note
The first five Figures of Merit are more general than last two FOMs. For example, plasma technology is often described in the literature by the conversion percentage and efficiency obtained by a particular plasma reactor configuration, and improving these two metrics can drive improvements in more general FOM's like specific energy consumption at the system level. However this "efficiency" is substantially different than the commonly reported "faradaic efficiency" for electrolytic technologies, because there is a fundamentally different physical process facilitating the chemical transformation, meaning that these metrics are not suitable for comparison. This is a huge problem in the literature, where different technological communities and disciplines report different kinds of efficiency metrics which may obscure the fundamental limits or benefits of the technology in comparison to others. The subtlety here is that to be compare relevant oxygen generation technologies, more general FOMs should be used to drive technological innovation.
Governing Equations
| Inputs | Key Relationships/Equations | Outputs |
|---|---|---|
| Plasma Energy Deposition Reactor Temperature Reactor Pressure Flow rate Enthalpy of Splitting |
simulations - experiments | Conversion Production Rate Conversion Efficiency Purity |
| Total System Power Production Rate |
SEC = P/r | Specific Energy Consumption |
| Specific Energy Consumption Production Rate Total System Mass System Lifetime required delta v |
LEE = SEC + (0.5*eta*m_s*v^2)/(rt) | Lifetime Embodied Energy |
All conversion processes must conserve matter, which is why the available resources constrain the products that can be made. Broadly speaking, all conversion processes require some energy. The first law of thermodynamics ensures that energy is conserved within a system ΔU=Q−W, where ΔU is the change in internal energy of the system, Q is the heat added to the system, and W is the work done on the system. The second Law introduces the concept of entropy (S) and sets limits on the efficiency of conversion processes. The entropy change in a system is: ΔS= Q/T, where T is the temperature at which heat is transferred. All conversion processes can be described by these laws, and fundamental limits on each depend on the mechanism by which the conversion is occurring. It depends on if it is a chemical reaction, or purely a thermal process involving a change of state, or something else.
Time Evolution of Figures of Merit
Because many space resource generation proposals require interplanetary travel to the Moon and Mars, at the market-level these technologies are in the initial proof-of-concept phase. The only demonstration of such a technology off-world is MOXIE (Mars OXygen ISRU Experiment) which produced oxygen aboard the perseverance rover [2]. But we have been using oxygen generation technologies in space on the ISS which can be modeled over time, though it is important to note that this technology is more advanced compared to other space resource generation systems. Additionally, there are currently very few adopters of this technology (because there are very few space stations), so there isn’t a lot of data.
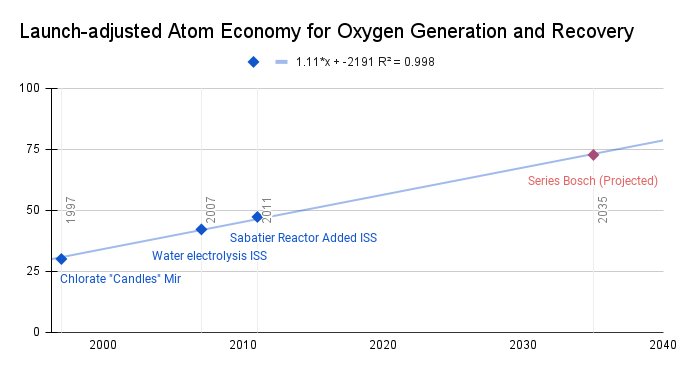
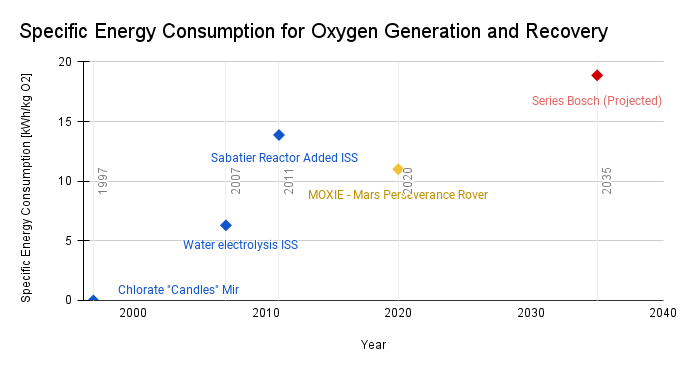
Increasing the Launch-adjusted atom economy FOM first became desirable due to the continuous operation of space stations like Mir and the ISS and the expensive cost of launch to supply life-support consumables. The first example is burning alkali chlorate “candles,” a form of solid oxide fuel generation [ https://en.wikipedia.org/wiki/Vika_oxygen_generator]: 60% of what is launched and used creates usable oxygen, so this leads to an atom economy of around 30%. Introducing water electrolysis to the ISS improved the atom economy, because 89% of what is launched is used as oxygen [3]. But because water is also a demand on the crew and this consumes water, it still has to be launched and results in an atom economy of 42%. An atom economy of 47% is achieved with a Sabatier reactor recovering some of the water and reducing the required launch mass, but still does not reach full conversion of CO2, and produces Methane which is currently vented [4]. A series bosch reactor, projected to reach maturity by around 2035, has a theoretical SAE limit of 73%, and paired with a carbon utilization technology could increase more [5]. Plotting the hypothetical maturation date, we see this may be the beginnings of stagnation, because where beyond full oxygen recovery there may be less desire to improve this FOM. The theoretical limit is 100%. In terms of oxygen generation and recovery technologies, it appears to be somewhere between rapid progress and slowing down (shown in the linear trend line) in improving how well we recycle launched oxygen-containing materials into space, approximately equal to about 1.11% improvement in LaAE per year using a linear regression.
The specific energy input required per kg of product has also increased over time, with the updated technologies and additions of reactors in series, but in the case of space station operations where energy can be renewably generated by solar panels, this has been favorable to minimize the launch mass. Interestingly, the decrease in specific energy input for MOXIE offers new value to stakeholders who are not concerned with full oxygen recovery, but instead want an efficient conversion process to scale up production.
Bibliography
[1] Scatteia, L., & Perrot, Y. (2021). Lunar market assessment: Market trends and challenges in the development of a lunar economy. PwC. Prepared by PwC Space Practice. Retrieved from www.pwc.fr/space.
[2] Laroque, D. A., Seó, S. T., Ayala Valencia, G., Laurindo, J. B., and Carciofi, B. A. M., “Cold plasma in food processing: Design, mechanisms, and application,” Journal of Food Engineering, Vol. 312, 2022, p. 110748. https://doi-org.ezproxyberklee.flo.org/10.1016/j.jfoodeng.2021.110748
[2] Lordos, G. C., Hoffman, J. A., and Summers, S. E., “Towards a Sustainable Industrial Development of Mars: Comparing Novel ISRU / ISM Architectures Using Lifetime Embodied Energy,” 2018 AIAA SPACE and Astronautics Forum and Exposition, 2018. https://doi-org.ezproxyberklee.flo.org/10.2514/6.2018-5125
[3] Hoffman, J. A., Hecht, M. H., Rapp, D., Hartvigsen, J. J., SooHoo, J. G., Aboobaker, A. M., McClean, J. B., Liu, A. M., Hinterman, E. D., Nasr, M., Hariharan, S., Horn, K. J., Meyen, F. E., Okkels, H., Steen, P., Elangovan, S., Graves, C. R., Khopkar, P., Madsen, M. B., Voecks, G. E., Smith, P. H., Skafte, T. L., Araghi, K. R., and Eisenman, D. J., “Mars Oxygen ISRU Experiment (MOXIE)—Preparing for Human Mars Exploration,” American Association for the Advancement of Science, Vol. 8, No. 35, 2022, p. eabp8636. https://doi-org.ezproxyberklee.flo.org/10.1126/sciadv.abp8636
[4] “Vika Oxygen Generator,” Wikipedia, Oct 06 2023. https://en.wikipedia.org/wiki/Vika_oxygen_generator
[5] Takada, K., Hornyak, D., Garr, J., Keuren, S. V., Faulkner, C., and ElSherbini, A., “Status of the Advanced Oxygen Generation Assembly,” presented at the 52nd International Conference on Environmental Systems, Calgary. https://ntrs.nasa.gov/citations/20230008013
[6] “SpaceCraft Oxygen Recovery (SCOR) - NASA.” Retrieved 8 October 2024. https://www.nasa.gov/spacecraft-oxygen-recovery-scor/