Difference between revisions of "Mining Critical Materials from Seawater and Brine"
| Line 4: | Line 4: | ||
==Roadmap Overview== | ==Roadmap Overview== | ||
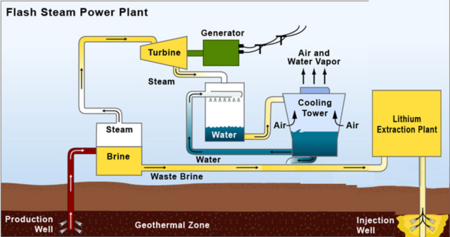
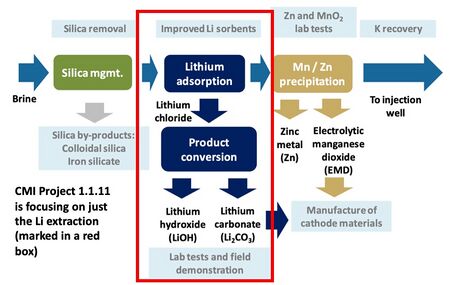
There are a variety of potential sources of lithium globally including minerals (e.g. clay, seawater, etc.); lithium-ion battery recycling; and saltwater brines (e.g. geothermal, continental, salt lakes, oil fields, etc.). We are focused on exploring lithium extraction from geothermal brines for a variety of reasons. One is that the concentration of lithium is higher in geothermal brines (approximately 300 ppm) than in other potential sources of lithium such as saltwater. Further, the Salton Sea in Southern California hosts a high concentration of lithium and is an attractive natural resource for the United States to take advantage of. Roughly 13 geothermal plants operate in this region with a combined electric generating capacity of 375 megawatts (MW), which together generate enough brine to recover a potential 200 metric tons of lithium per year - enough to supply the global demand for lithium. We are roadmapping a sorbent technology that extracts lithium from geothermal brines with a focus on how this technology can be applied to geothermal plants in Southern California. The two graphics below break out the schematic and R&D boundaries for the sorbent technology. | |||
[[File:Mineral extraction plant concept.png| | [[File:Mineral extraction plant concept.png|450px]] | ||
[[File: Research tasks.jpg | | ''Concept of mineral extraction plant utilizing post-power production, pre-injection geothermal brine.''<ref>Paranthaman, M. P., Ling, L., Luo, J., Hoke, T., Ucar, H., Moyer, B., and Harrison, S. “Recovery of lithium from geothermal brine with lithium-aluminum double hydroxide chloride sorbents.” Environmental Science & Technology, 51: 13481-13486. 2017.</ref> | ||
[[File:Research tasks.jpg|450px]] | |||
''Schematic of R&D tasks.''<ref>Paranthaman, M. P. “Lithium extraction from geothermal brine solution.” Webinar presented by the U.S. Department of Energy Oak Ridge National Laboratory.” 2018. Available at: https://iastate.app.box.com/v/cmi-webinar-november-2018</ref> | |||
==Design Structure Matrix (DSM) Allocation== | |||
==Roadmap Model using OPM== | |||
[[File: LiOPD.png| LiOPD.png]] | |||
==Figures of Merit== | |||
{| class="wikitable" | |||
|- | |||
! FOM Name !! Unit !! Description | |||
|- | |||
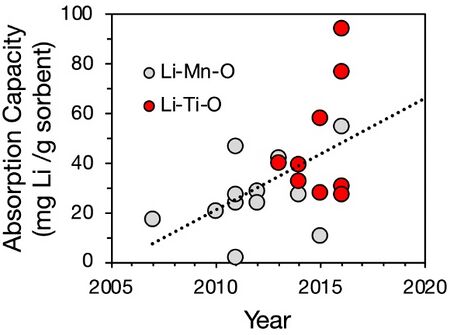
| Adsorption capacity || [mg/g] || | |||
* Milligrams of Lithium per gram sorbent. | |||
* 6 mg/g is an achievable target. Higher capacities of ~25-30 mg/g are achievable but present a cost tradeoff because achieving higher adsorption capacity requires lower pH levels, and adjusting the solution pH adds costs. | |||
|- | |||
| Selectivity || [dmnl] || | |||
* Dimensionless quantity that describes the concentration of Lithium and other minerals in filtered fluid. | |||
* A high concentration of lithium and low concentration of contaminants (i.e. sodium, potassium) is optimal. | |||
|- | |||
| Lithium Production Cost || [$/kg] || | |||
* In evaluating the techno-economic performance of various lithium extraction methods (i.e. seawater extraction, mining from ores), it is crucial to compare costs on an apples-to-apples basis with existing production technologies. The production cost in $/kg illustrates the sum cost of Lithium production per unit quantity of Lithium. | |||
* The Lithium Production Cost by necessity includes underlying cost components related to the Lithium sorbent itself that will be the basis of further exploration, such as the cost of sorbent materials, manufacturing, operations & management, etc. | |||
|} | |||
[[File:Adsorption capacity.jpg|450px]] | |||
''Adsorption capacities of sorbents.''<ref>Ref</ref> | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Revision as of 12:40, 1 October 2020
Technology Roadmap Sections and Deliverables
This technology roadmap is given the unique identifier:
Roadmap Overview
There are a variety of potential sources of lithium globally including minerals (e.g. clay, seawater, etc.); lithium-ion battery recycling; and saltwater brines (e.g. geothermal, continental, salt lakes, oil fields, etc.). We are focused on exploring lithium extraction from geothermal brines for a variety of reasons. One is that the concentration of lithium is higher in geothermal brines (approximately 300 ppm) than in other potential sources of lithium such as saltwater. Further, the Salton Sea in Southern California hosts a high concentration of lithium and is an attractive natural resource for the United States to take advantage of. Roughly 13 geothermal plants operate in this region with a combined electric generating capacity of 375 megawatts (MW), which together generate enough brine to recover a potential 200 metric tons of lithium per year - enough to supply the global demand for lithium. We are roadmapping a sorbent technology that extracts lithium from geothermal brines with a focus on how this technology can be applied to geothermal plants in Southern California. The two graphics below break out the schematic and R&D boundaries for the sorbent technology.
Concept of mineral extraction plant utilizing post-power production, pre-injection geothermal brine.<ref>Paranthaman, M. P., Ling, L., Luo, J., Hoke, T., Ucar, H., Moyer, B., and Harrison, S. “Recovery of lithium from geothermal brine with lithium-aluminum double hydroxide chloride sorbents.” Environmental Science & Technology, 51: 13481-13486. 2017.</ref>
Schematic of R&D tasks.<ref>Paranthaman, M. P. “Lithium extraction from geothermal brine solution.” Webinar presented by the U.S. Department of Energy Oak Ridge National Laboratory.” 2018. Available at: https://iastate.app.box.com/v/cmi-webinar-november-2018</ref>
Design Structure Matrix (DSM) Allocation
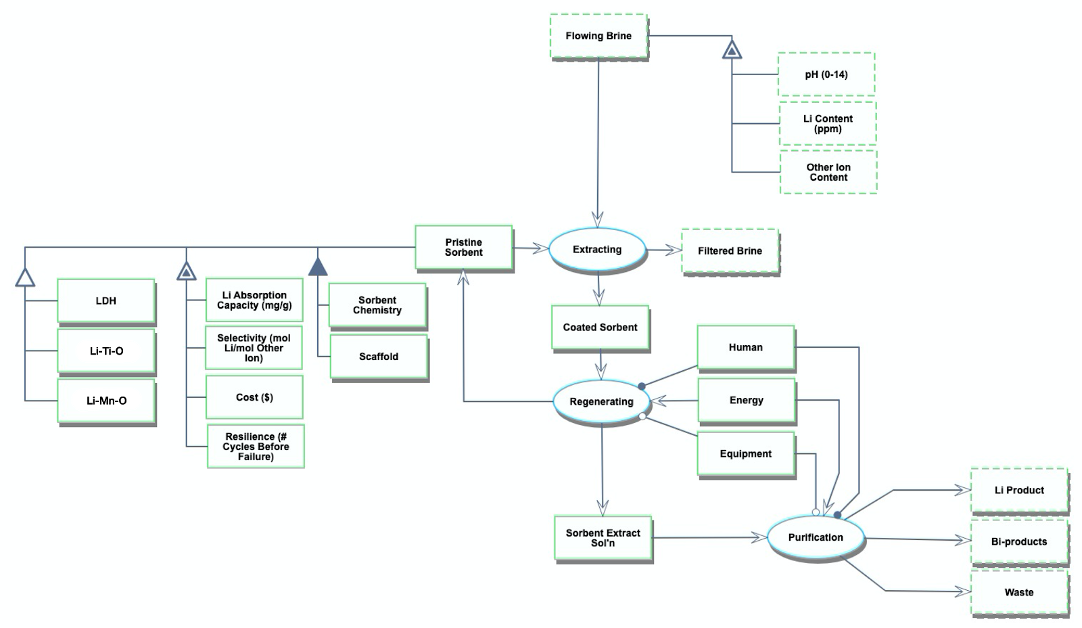
Roadmap Model using OPM
Figures of Merit
| FOM Name | Unit | Description |
|---|---|---|
| Adsorption capacity | [mg/g] |
|
| Selectivity | [dmnl] |
|
| Lithium Production Cost | [$/kg] |
|
Adsorption capacities of sorbents.<ref>Ref</ref>
References
<references />